image:
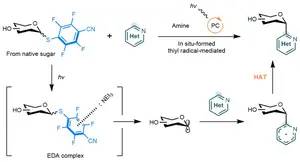
Schematic illustration of the one-step activation of native sugars followed by photocatalytic coupling with N-heteroarenes to deliver C-heteroaryl glycosides. The sugar compound is first selectively activated at the anomeric (C1) position, then exposed to an amine, a photocatalyst and visible light, allowing it to form a new carbon–carbon bond with a nitrogen-containing aromatic molecule. This streamlined process avoids many of the usually required protective steps.
Credit: Nature Synthesis
Researchers from the National University of Singapore (NUS) have developed a “capping-and-coupling” strategy to transform naturally occurring (native) sugars directly into compounds known as C-heteroaryl glycosides. This makes it easier to produce such molecules that are valuable for drug and vaccine development.
The research team was led by Associate Professor KOH Ming Joo from the NUS Department of Chemistry and Professor CHAN Chun Yong Eric from the NUS Department of Pharmacy and Pharmaceutical Sciences. The research breakthrough was published in the scientific journal Nature Synthesis on 19 January 2026.
C-Heteroaryl glycosides are found in many medicinally relevant molecules with interesting biological activities and are used in the development of effective mRNA vaccines against COVID-19 and other diseases. In their physiologically active forms, these compounds are densely functionalised with nitrogen-containing aromatic groups and numerous hydroxyl (-OH) groups. However, the chemical synthesis of C-heteroaryl glycosides is challenging and typically involves many steps, including hydroxyl protection and deprotection and the use of harsh reagents. This makes the process time-consuming, wasteful, and difficult to apply on a larger scale.
Assoc Prof Koh said, “The most appealing way to make C-heteroaryl glycosides and create new functional molecules is to merge naturally occurring native saccharides with N-heteroarenes, both of which are prevalent in nature, through direct carbon-carbon bond formation.”
The researchers designed a “capping-and-coupling” concept that transforms native sugars into C-heteroaryl glycosides in one step. The approach works by temporarily “capping” the sugar at the anomeric (C1) position, which selectively activates it for further reaction. This generates a bench-stable, redox-active intermediate that can then be joined to a nitrogen-containing aromatic group through light-driven (photocatalytic) carbon–carbon bond formation. Importantly, this reaction proceeds through a previously unexplored chemical pathway, distinct from existing photochemical methods used to activate sugars. As a result, the method enables simple and efficient coupling of a wide range of single and multi-sugar molecules with diverse nitrogen-containing aromatics.
Beyond method development, the researchers also tested some of the resulting compounds and found one C-heteroaryl glycoside that strongly slows glycogen breakdown, about 114 times more potent than its parent sugar scaffold. This highlights the compound’s potential for metabolic and therapeutic applications.
“We believe that this new methodology offers a powerful avenue for late stage glycosylations of complex molecules and to build innovative, functional carbohydrates,” added Assoc Prof Koh.
Studies are ongoing to extend the capping-and-coupling strategy to synthesise other important classes of carbohydrates for biological applications.
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Photocatalytic coupling of unprotected sugars and N-heteroarenes
Article Publication Date
19-Jan-2026
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.