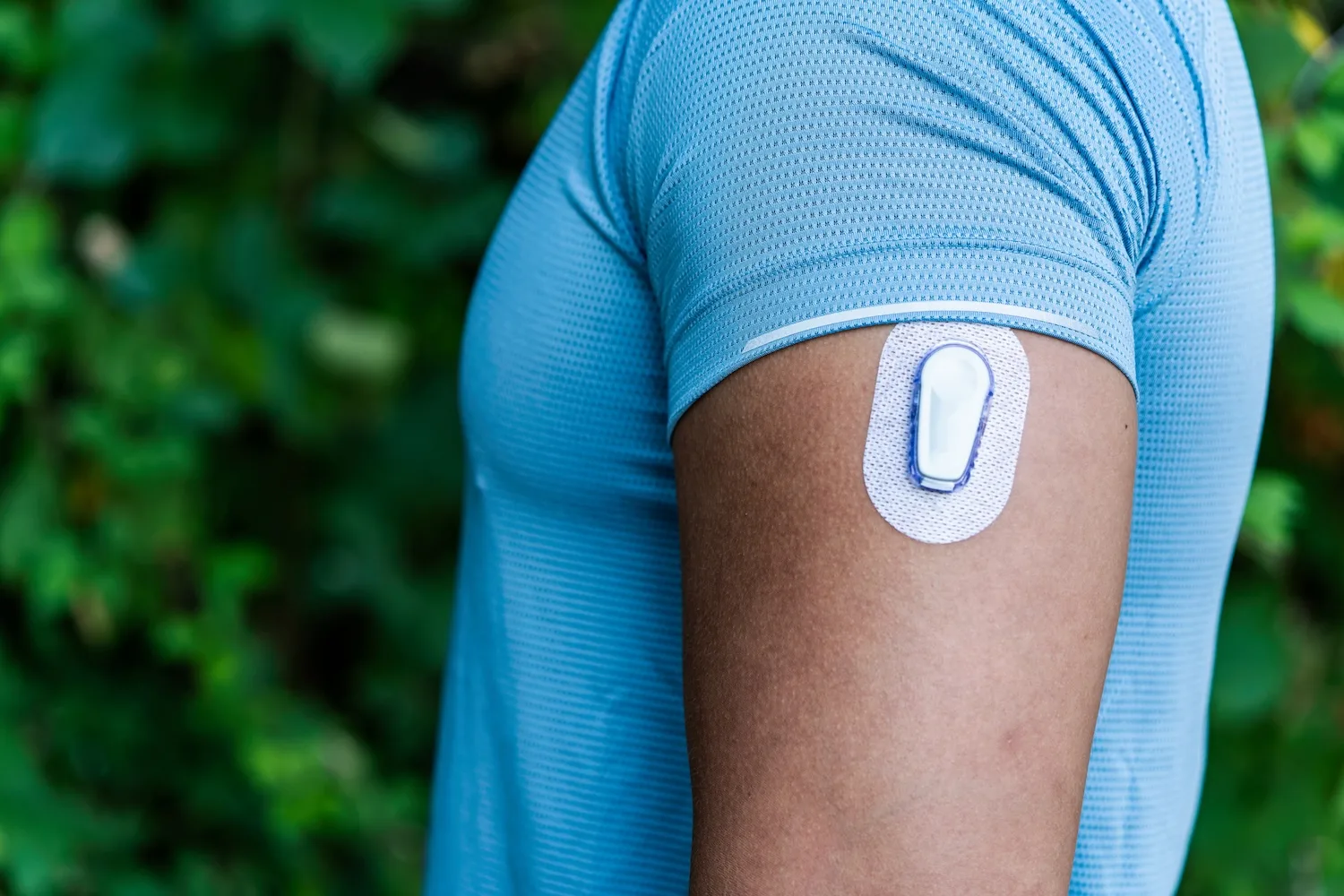
The FDA has issued a warning regarding a labeling issue affecting several of Trividia Health’s True Metrix blood glucose monitoring systems that could delay treatment during episodes of severe hyperglycemia.
Trividia issued a correction in a Feb. 6 letter to customers covering all models of its True Metrix systems, according to a Feb. 16 FDA early alert. A labeling update clarifies instructions tied to the E-5 error code, which may indicate a blood glucose reading above 600 mg/dL or a test strip error.
The affected products include the True Metrix, True Metrix Air, True Metrix Go and True Metrix Pro systems.
Trividia said users who receive an E-5 code and experience symptoms of high blood sugar — such as fatigue, thirst, frequent urination or blurred vision — should seek immediate medical attention. Previous instructions may have contributed to delays in treatment in critical situations.
As of Jan. 16, Trividia has reported 114 serious injuries and one death associated with the issue. The FDA said it is continuing to review the matter and will update its public alert as new information becomes available.