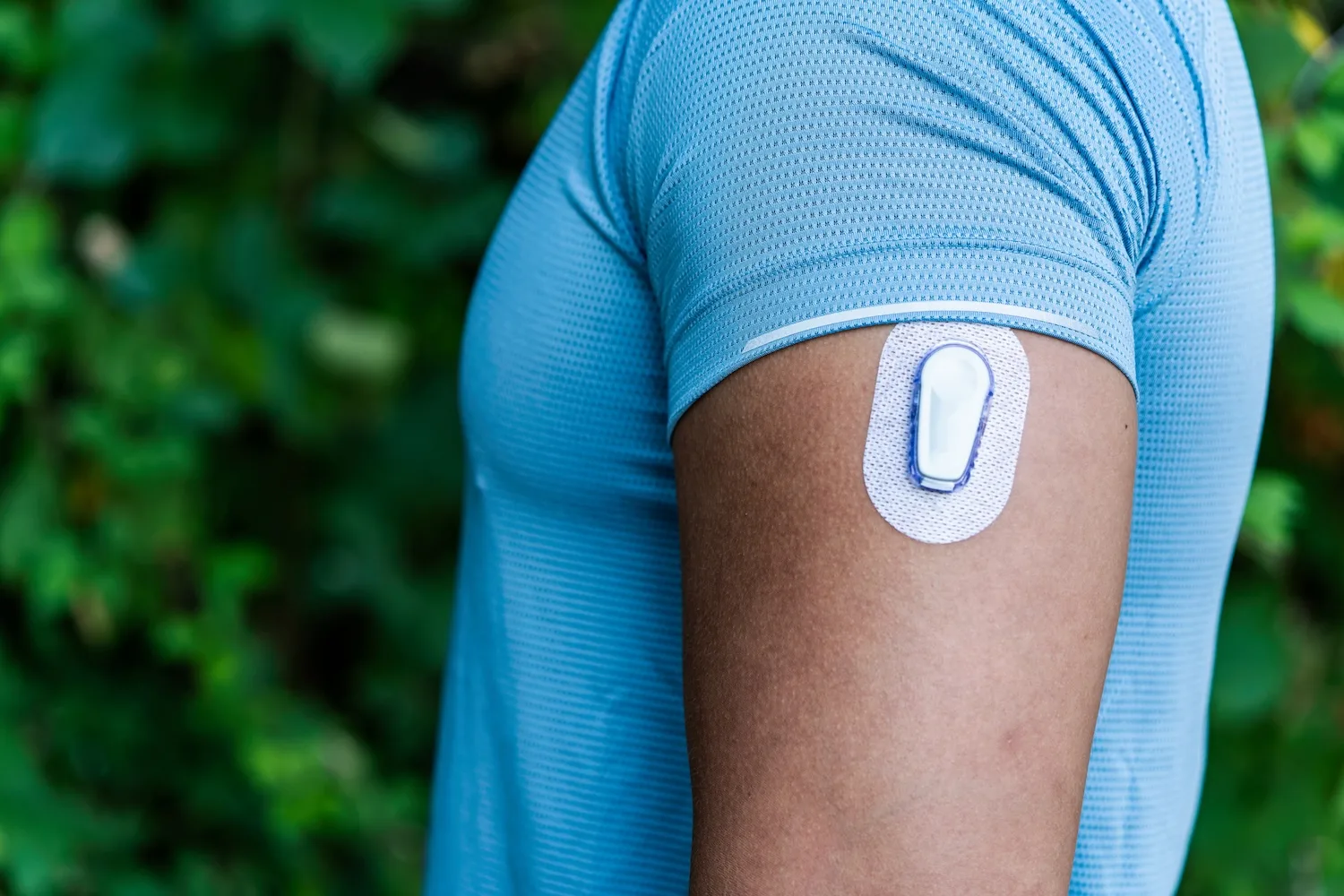
Abbott Diabetes Care is recalling certain FreeStyle Libre 3 and FreeStyle Libre 3 Plus sensors due to falsely low glucose readings.
The FreeStyle Libre 3 and FreeStyle Libre 3 Plus are continuous glucose monitoring (CGM) devices indicated for the management of diabetes in patients aged 4 years and older. These real time CGM devices replace traditional finger prick tests by monitoring trends and patterns to aid in the detection of hyper- and/or hypoglycemic episodes and to optimize therapy.
According to the Food and Drug Administration (FDA) safety alert, the sensors are providing incorrect low glucose readings, that if gone undetected for a prolonged period of time, may result in a mismanagement of diabetic therapy and serious complications.
As of January 7, 2026, Abbott Diabetes Care has reported 860 serious injuries and 7 deaths related to this recall. The Agency has designated this issue as a Class I Recall, indicating a high risk of serious health consequences or death.
The recall involves the following sensors:
- FreeStyle Libre 3 Sensor
- Model Numbers: 72081-01, 72080-01
- Unique Device Identifiers: 00357599818005, 00357599819002
- FreeStyle Libre 3 Plus Sensor
- Model Numbers: 78768-01, 78769-01
- Unique Device Identifiers: 00357599844011, 00357599843014
The full list of affected lots for products within the US and those outside of the US can be found here.
The recall does not affect FreeStyle Libre 3 readers or mobile apps and does not include other Libre products such as the FreeStyle Libre 14 day, FreeStyle Libre 2, FreeStyle Libre 2 Plus, or Libre Pro sensors.
The Company requests all health care providers inform patients of this issue and instruct patients to visit www.FreeStyleCheck.com to verify their sensors by inputting the sensor serial number. Patients may locate the product serial number in the app, on the reader, or on the sensor packaging. If a sensor is confirmed as affected, patients should immediately discontinue use and dispose of the sensor. Upon confirmation, the website will prompt patients to enter their contact information so a replacement product can be sent to them at no cost.
To provide further clarity on the situation, Abbott shared the following message:
“Abbott initiated a medical device correction on November 24, 2025 for certain FreeStyle Libre 3 and FreeStyle Libre 3 Plus sensors in the US after internal testing determined that some of these sensors may provide incorrect low glucose readings. We immediately contacted customers to make them aware and provide support.
Abbott has identified and resolved the cause of the issue, which relates to one production line among several that make Libre 3 and Libre 3 Plus sensors. The company continues to produce Libre 3 and Libre 3 Plus sensors to fulfil replacement and new orders and does not expect significant supply disruptions.”
Individuals with recall related inquiries can contact Abbott Diabetes Care at 1-833-815-4273 or https://www.freestyle.abbott/us-en/support/contact-us.html. Adverse reactions should be reported to the FDA’s MedWatch Adverse Event Reporting program.
Editor’s Note: This article was updated on February 5, 2026 to include a response from Abbott.
This article originally appeared on MPR